A research team from the University of Oxford conducted hypoxemia detection and sleep monitoring on 50 hospitalized SDB patients for 7 days from November 2022 to January 2023. Research results show that Viatom pulse oximeter are highly accurate and reliable, can effectively capture and record patients’ hypoxemia events and sleep disorders, and are well compared with traditional hospital equipment.
With rigorous procedures, the research analyzed the signal quality, consistency, and fidelity of the SpO2 readings under various physiological conditions. After comparing the findings against the gold standard benchtop oximeters, the outcomes substantiated the Viatom Checkme O2+ was within that required by the International Organization for Standardization guidelines.
Checkme O2+ exhibited consistent performance and dependability in detecting and monitoring oxygen saturation levels. Currently, Checkme O2 series wrist oximeters have been widely used in various clinical and non-clinical scenarios, including wearable monitoring, sleep monitoring, continuous care monitoring, etc.

Introduction to the Testing Methods of Research
In this part, let’s discover the testing procedures accompanying the experiment.
Motion Tasks
In the empirical design of the study, subjects were engaged in seven motion tasks, including rest, sit-to-stand, tapping, rubbing, drinking, turning pages, and utilizing a tablet. It was employed to assess the operational robustness of the wrist oximeter under dynamic physiological conditions. The consequence shows that CheckmeO2 + ensures the resultant SpO2 readings were not compromised by artifacts or motion-induced noise for the device’s resilience and consistency in real-world, non-static environments.
Hypoxia Exposure
A controlled hypoxia exposure protocol was administered, wherein subjects underwent incremental reductions in inspired oxygen levels for designated arterial SaO2 benchmarks: 100%, 95%, 90%, 87%, 85%, 83%, and 80%. The approach facilitated an inclusive evaluation of the wrist oximeter’s precision and responsiveness across a spectrum of oxygen saturation levels. Hence, this establishes its diagnostic accuracy and adaptability in fluctuating hypoxic conditions.
Sample Data Collection
Collect the subject’s sample data, including age, gender, skin type, baseline heart rate and SaO2, etc. After collection researchers conducted a comparative analysis between the peripheral SpO2 estimates yielded by the wrist oximeters and simultaneous SaO2 samples obtained invasively. This relative strategy aimed to ascertain the conformity of the oximeters’ readings with reference SaO2 values, which enables a detailed evaluation of the device’s capability for accurate and clinically reliable SpO2 estimations.
Root-Mean-Square Error (RMSE)
According to ISO guidelines, the accuracy of an oximeter’s SpO2 estimate is determined by the root mean square error (RMSE) between the measured value (SpO2i) and the reference value (SaO2i):

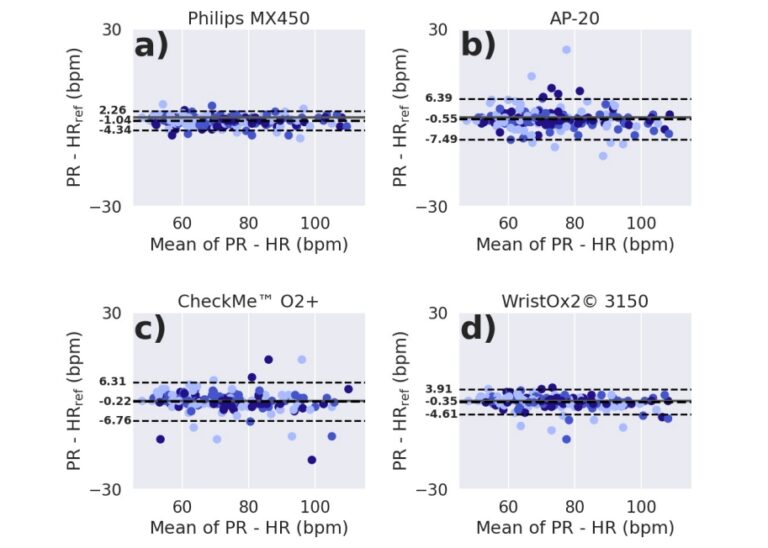
RMSE is the core of the quantitative measure of the wrist oximeter’s accuracy.It offers statistical insight into the magnitude of deviation and consistency of the device’s SpO2 readings in a clinical diagnostic context. After calculated, Checkme O2+ overall RMSE below 4% (and below 8% when considering the 95% CI; Table 3), meeting the ISO 80601-2-61:2019 requirement.
Result
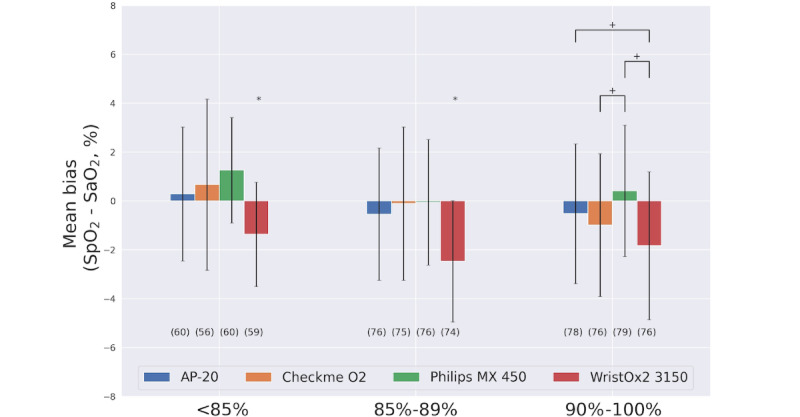
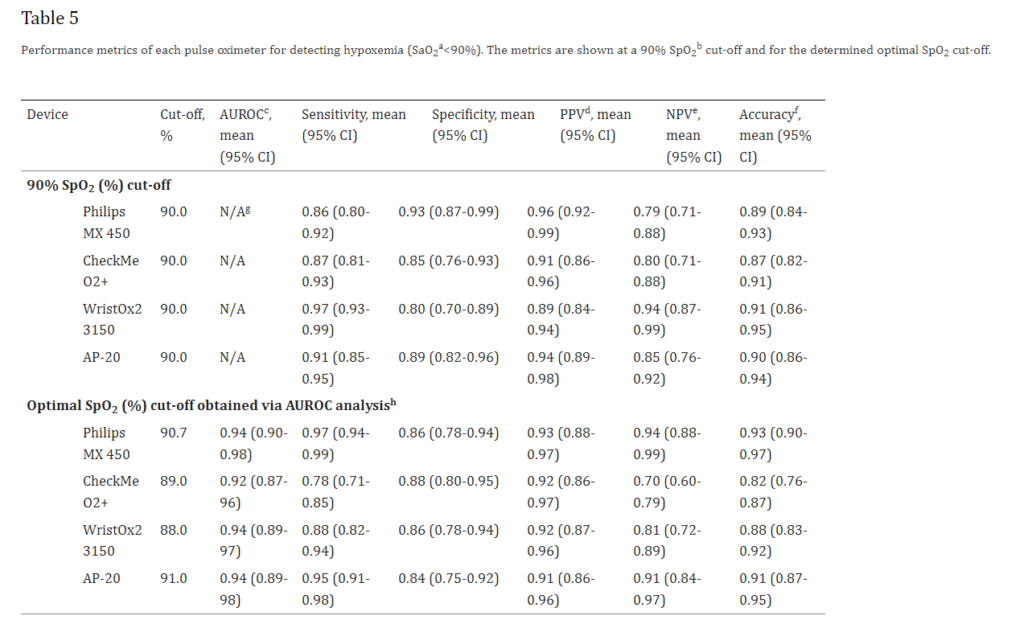
After analyzing 215 sample data from 33 participants, the mean bias of CheckmeO2+ during the hypoxic exposure phase was -0.22, significantly better than other oximeters in the trial. When SaO2<90%, the sensitivity of CheckmeO2+ is 0.87 and the specificity is 0.85. When SAO2<85, no obvious error is seen, which complies with the requirements of ISO 80601-2-61:2019.
Blood oxygen saturation (SpO2) is an important measurement indicator for monitoring patients with acute and chronic diseases related to low blood oxygen levels. As long-term monitoring of blood oxygen at home has become a necessary monitoring method for chronic disease management, wearable blood oximeter offers new opportunities for RPM and continuous monitoring.
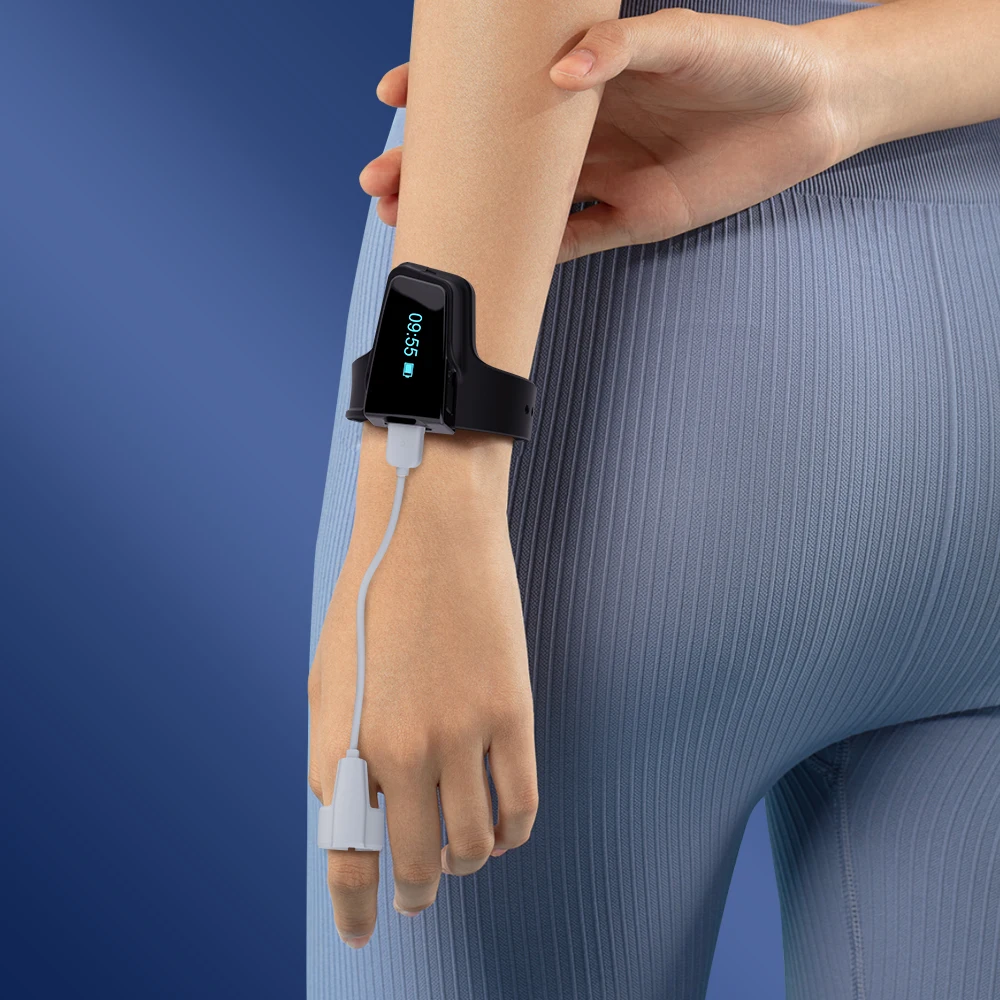
Obstructive Sleep Apnea (OSA) and Hypoxia
Sleep apnea is a common cause of hypoxemia. Sleep apnea refers to a sudden pause in breathing during sleep. Clinically, breathing pauses of more than 10 seconds are usually regarded as a sleep breathing event. According to the frequency of sleep breathing events, it is clinically divided into mild, moderate and severe sleep apnea.
This interplay between OSA and hypoxia emphasizes precise and continuous oxygen saturation monitoring, wherein the application of wrist oximeters manifests as a key diagnostic asset in capturing the multifaceted dynamics of oxygen desaturation associated with OSA.
Common Diagnostic Symptoms of OSA
Snoring, daytime sleepiness, and observable pauses in breathing characterize the symptomatic presentation of OSA. Additionally, individuals may exhibit cognitive impairments like difficulties with memory and concentration, compounded by emotional volatility expressed as unusual moodiness or irritability. Morning headaches and dry mouth further augment the diagnostic symptomatology, converging to form a complete clinical picture for diagnostic consideration.
Remember, the symptomatic diversity requires a multifaceted diagnostic approach to encapsulate the intricate manifestations of OSA in a clinically coherent manner.
Diagnosis Process of OSA
The diagnostic pathway of OSA is orchestrated, which commences with a physical examination to identify any anatomical predispositions conducive to airway obstruction and increase the specificity of questionnaire diagnosis. Subsequently, polysomnography is an essential diagnostic tool. It enables the assessment of sleep architecture and associated respiratory parameters, adopting an environment of diagnostic precision.
Home sleep apnea testing further improves the diagnostic continuum. It offers a practical modality to determine the presence and severity of OSA within the domiciliary comfort. The integration of wrist oximeters within the diagnostic algorithm increases the granularity of oxygen saturation assessments for a refined diagnostic resolution helpful for targeted therapeutic strategies.
Importance of OSA Early Detection
Emphasizing early detection of OSA and hypoxia is crucial. It necessitates a strategic confluence of diagnostic innovation and clinical acumen for timely intervention and progressive pathological sequelae. The evolution of wearable diagnostic devices like wrist oximeters represents a mainstream tech convergence, imbued with practical utility to address this diagnostic imperative.
These devices embody precision and convenience and augment the capacity for real-time insights into oxygen saturation dynamics. So, it encourages the diagnostic framework with heightened responsiveness and adaptability. The pragmatism imbued within wearable diagnostic technologies validates their integral role in piloting the diagnostic complexities inherent to OSA and hypoxia. It aligns technological development with clinical exigency to boost patient outcomes.
Viatom Checkme™ O2 Max
The tech background of wrist oximeters has grown with the introduction of Viatom Checkme™ O2 Max. Vitally, it is an upgraded iteration of the Viatom Checkme O2+, the best pulse oximeter for overnight monitoring. It signifies a tactical augmentation of device functionalities and features.

Upgraded Battery
The battery life of the Viatom Checkme™ O2 Max has undergone optimization while transitioning from a 16-hour to 72-hour operational durability following a single charge. Such a modification not only curtails the interruptions attributed to frequent charging but also boosts the device’s capacity for prolonged and continuous monitoring sessions. For instance, the superior battery life guarantees uninterrupted data collection and analysis in a clinical scenario requiring extended patient monitoring. Thus, it improves diagnostic accuracy and patient management policies.
More Frequent Data Collection
Viatom Checkme™ O2 Max shows an advancement in data collection frequencies, where recording intervals have been reduced from every 4 seconds to every 2 seconds. It fortifies the wrist oximeter’s capability to curate a more detailed dataset for analytically resolving collected physiological parameters. For instance, such a feature is pivotal in capturing subtle physiological fluctuations for refining diagnostic assessments and therapeutic interventions.
Design for Remote Monitoring
Viatom Checkme™ O2 Max’s wrist oximeter encompasses advanced reporting capabilities. It promotes a dynamic integration of data analysis, remote conduction, and management functionalities. The device facilitates detailed analytical reporting for interpretative clarity and actionable insights derivable from the collected data. In practical terms, such a feature augments the capacity for remote patient monitoring. It aids in the streamlined management of diagnostic and therapeutic protocols. Meanwhile, it adjusts patient care and outcome trajectories.
Built-in Smart Vibrator
Incorporating an intelligent vibrator functionality within the Viatom Checkme™ O2 Max exemplifies an innovative approach to real-time user engagement and response facilitation. Specifically engineered to be triggered by instances of oxygen desaturation, the vibrator confirms immediate alert dissemination. It nurtures an environment for prompt user reaction and adjustment in response to detected physiological abnormalities. Such a feature enhances the proactive management of conditions where timely user responsiveness helps alleviate potential health risks.
Gentle Vibrating Alert
The device’s design philosophy expresses a promise of user-centric innovation, as reflected in the implementation of a gentle vibrating alert mechanism. The feature is calibrated to emit subtle vibratory signals in detected physiological deviations for a non-disruptive yet effective alerting strategy. It enriches the wrist oximeter’s usability, mainly in sleep monitoring contexts where keeping a favorable sleep environment is essential for accurate data collection and analysis.
Our Vision and Mission
We’re profoundly grateful for Oxford University’s discerning approval, which reflects an evaluation of our wrist oximeter’s capabilities. Essentially, Viatom’s innovative vision orbits around using advanced photoplethysmography and algorithms to detect and analyze blood oxygen saturation and heart rate variability. Our wrist oximeter expedites precise biometric data acquisition in real time in the complex interplay of hardware and software. Accordingly, it amplifies its utility in different, dynamic, and demanding health monitoring scenarios.
Consequently, while nurturing smooth integration into clinical workflows and home monitoring regimes, it seeks to set transformative benchmarks in delivering accurate and actionable bodily insights. This authenticates its role as an influential tool in preemptive health management and informed clinical decision-making. The premeditated alignment not only underscores our obligation to technological finesse but also encourages our pursuit of excellence in cultivating state-of-the-art solutions that resonate with the embryonic paradigms of healthcare and well-being.
Conclusion
Viatom is a leader in updating and improving health monitoring technologies, exemplified by products like our wrist oximeter. Our devices, infused with technical mastery and user-focused design, excel in performance, usability, and exactness. Viatom emphasizes the synchrony between technological excellence and practical applicability, which allows users to experience superior standards of health monitoring. We impeccably blend technical dexterity with suitable health monitoring needs while establishing ourselves as a model of innovation.
Reference:
Santos M, Vollam S, Pimentel MA, Areia C, Young L, Roman C, Ede J, Piper P, King E, Harford M, Shah A, Gustafson O, Tarassenko L, Watkinson P. The Use of Wearable Pulse Oximeters in the Prompt Detection of Hypoxemia and During Movement: Diagnostic Accuracy Study. J Med Internet Res. 2022 Feb 15;24(2):e28890. doi: 10.2196/28890. PMID: 35166690; PMCID: PMC8889481.